When fireworks light up the night sky with bursts of vivid colors and spectacular shapes, it's natural to wonder exactly how are metallic salts used in fireworks to create these breathtaking effects.
At Red Apple Fireworks, we’re passionate about bringing excitement and joy to celebrations everywhere—from backyard DIY displays to large holiday events.
Knowing the chemistry behind these dazzling shows enriches the experience for everyone watching.
Today, we’ll dive into the fascinating chemistry of fireworks to explore how metallic salts transform ordinary fireworks into extraordinary displays!
What this article covers:
- What Are Metallic Salts and How Do They Create Color?
- Key Metallic Salts and Their Colors
- How Metallic Salts Create Brilliant Firework Colors
- Safety and Environmental Considerations of Metallic Salts
What Are Metallic Salts and How Do They Create Color?
When it comes to creating colour in fireworks, metallic salts are the true stars of the show.
These compounds—made by combining metals with non-metals like chlorine or carbonates—are the key ingredients responsible for the stunning reds, blues, greens, and yellows you see bursting across the sky.
So, how are metallic salts used in fireworks? They’re mixed into small pellets called “stars” inside each firework shell.
When the firework is ignited, these stars are heated to extremely high temperatures, which excites the electrons within the metallic salts.
As those electrons return to their original energy levels, they release visible light—each metal emitting its own distinct colour.
For example:
-
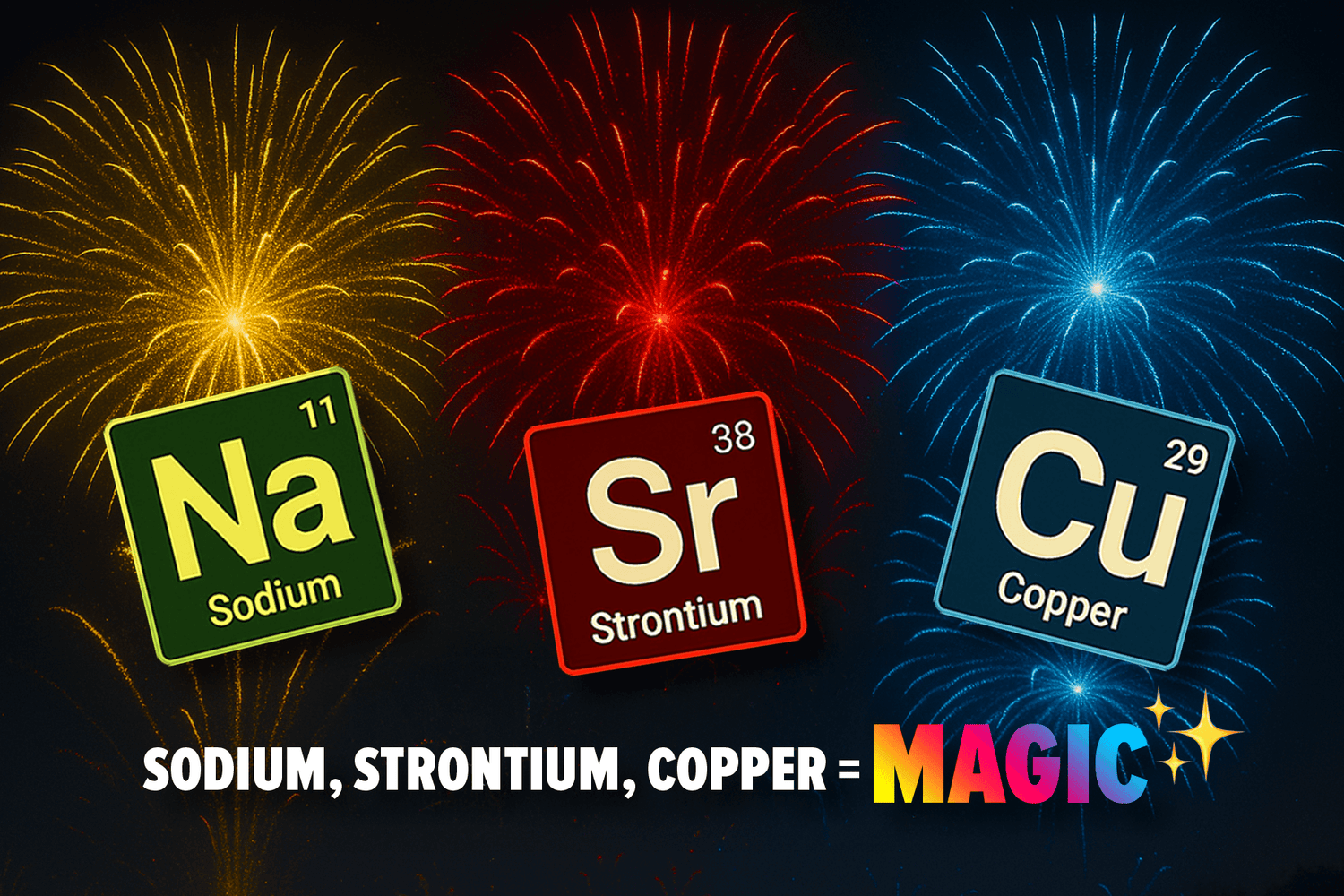
Copper salts create brilliant blues and greens
-
Strontium compounds burn a deep, bold red
-
Sodium salts produce vivid yellow or golden hues
Without metallic salts, fireworks would be little more than white or dull yellow flashes. By understanding the chemistry behind how they work, we can better appreciate the vibrant art and precision of every firework display.

Key Metallic Salts and Their Colors
At Red Apple Fireworks, we specialize in offering fireworks packed with precisely selected metallic salts to guarantee stunning colors and consistent quality.
Here’s a quick guide to some key metallic salts you'll encounter:
Sodium and Yellow Flames
Sodium salts, specifically sodium nitrate or sodium chloride, produce vibrant yellow flames. If you've ever marveled at a sparkling golden-yellow explosion on July 4th, thank sodium!
Strontium for Red Flames
Strontium salts, especially strontium carbonate and strontium nitrate, are responsible for intense reds. They're perfect for patriotic displays or romantic events, ensuring an unforgettable, vibrant red spectacle in the sky.
Copper for Blue Flames
Copper salts like copper chloride or copper oxide deliver mesmerizing shades of blue.
Among the most challenging colors to perfect in fireworks, achieving that pure blue flame is a mark of true pyrotechnic mastery—and Red Apple Fireworks proudly offers fireworks that produce dazzling blues every time.
How Metallic Salts Create Brilliant Firework Colors
Once a firework is in the air, it’s the chemistry inside the stars that brings colour to life. Each star contains carefully chosen metallic salts—compounds that react to heat and emit distinct wavelengths of light. These reactions are what create the rainbow of colours lighting up the night sky.
At Red Apple Fireworks, we use only high-quality salts to deliver vivid, reliable hues. Here’s a quick look at how some of the most popular colours are made:
- Copper Compounds: Create rich blues and vibrant greens. Depending on the temperature and formulation, copper carbonate can yield green, while copper chloride produces deep, oceanic blue.
- Strontium Salts: Responsible for intense reds. These salts burn consistently bright, making them ideal for everything from romantic displays to bold patriotic bursts.
- Sodium Compounds: Generate warm yellow and golden tones. Sodium nitrate and sodium oxalate are used for their stable, sunny glow that cuts through even the brightest display.
The art of fireworks comes down to balance. Too much of one element—or the wrong burn temperature—can throw off the entire colour. That’s why precision chemistry is at the heart of every Red Apple firework.

Safety and Environmental Considerations of Metallic Salts
At Red Apple Fireworks, safety and environmental care are crucial. Understanding the chemistry behind metallic salts also means considering safe handling practices and environmental impact.
When handling fireworks, always follow safety guidelines. Store fireworks in cool, dry places, away from flames or sparks.
Use protective gear when lighting fireworks and keep spectators at a safe distance. Environmentally friendly fireworks minimize heavy metal content and residue, protecting our environment without sacrificing color or quality.
Conclusion
Now you know exactly how metallic salts are used in fireworks—the chemistry responsible for the breathtaking colors that make every fireworks show unforgettable.
At Red Apple Fireworks, we're proud to offer top-quality fireworks with vibrant colors and outstanding effects, perfect for DIY enthusiasts, family celebrations, holiday events, and professional planners alike.
From our missiles fireworks to family-friendly favorites, we’re here to brighten every special moment.
From July 4th celebrations to weddings, birthdays, or just because—make every event magical with Red Apple Fireworks!
If you want to learn more, check out our articles below:
- What Makes Fireworks Whistle
- Is Magnesium Used in Fireworks
- How Do Fireworks Get Their Color?
- What Element Makes Purple Fireworks?
- What Element Will Give Fireworks Their Yellow Color?
- What Makes Fireworks Blue?
- How Does a Firework Work?
- What Chemicals Are Used in Fireworks?
- What Minerals Are Used to Make Gold Sparks Fireworks?
- How Are Fireworks Made?
- Where Are Fireworks Made?
- Is There Gunpowder in Fireworks?
- How to Light Fireworks
- Can You Do Fireworks in the Rain?
- How Long Do Fireworks Last?
-
What Causes Fireworks?











Leave a comment
All comments are moderated before being published.
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.